Physics - Grade XII or Standard XII
Chapter 17: Electrons and Photons
Photoelectric effect:
The phenomenon of emission of electrons by certain metals, when it is exposed to radiations of suitable frequencies is called as photoelectric effect and emitted electrons are called photoelectrons.
Photoelectrons:
Electrons emitted in photoelectric effect are called photoelectrons.
Einstein’s assumptions:
Einstein made two assumptions while explaining phenomenon of photoelectric effect, as follows:
(a) A radiation of frequency ν consists of a stream of discrete quanta or photons and energy of each photon is hν, where h is Planck’s constant. The photons move through space with the speed of light,
(b) When radiation of frequency ν is incident on photosensitive metal surface, there are collisions between the photons and the free electrons in metal. During such collision entire energy of a photon is transferred to an electron. If this energetic electron now possesses sufficient energy then it comes out of the metal surface as photoelectron.
Click Here to Go To Top of The Page
Einstein’s photoelectric equation:
According to Einstein, when a photon of energy hν is incident on a photosensitive metal, it is absorbed by an electron in that metal, this electron acquires the energy hν, and photon simply disappears. This energetic electron comes out of the metal surface as photoelectron. This electron has to spend the energy φ0 while coming out of the surface to overcome the potential barrier and remaining energy appears as kinetic energy of electron. Einstein’s photoelectric equation can be stated as follows:
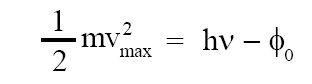
where, m = mass of photoelectron, vmax = maximum speed of photoelectron, h = Planck’s constant, ν = frequency of incident photon, φ0 = work function of metal, L.H.S. of above equation = maximum kinetic energy of emitted electron, hν = energy of incident photon.
Threshold frequency (ν0):
The minimum frequency of photon required to cause photoelectric emission of photoelectron from a given metal is called threshold frequency of that metal and is denoted by ν0.
Click Here to Go To Top of The Page
Characteristics of photoelectric effect:
Characteristics of photoelectric effect are as follows:
(i) For a given photosensitive metal, there exists a certain minimum cut-off frequency of the incident radiation, called threshold frequency ν0, below which no emission of photoelectrons take place. The threshold frequency is different for different metals.
(ii) For a given photosensitive metal and frequency of incident radiation (above threshold frequency ν0), the photoelectric current is directly proportional to the intensity of incident light. More the intensity of incident light, means more the number of incident photons, more the number of emitted photoelectrons, and hence more the photocurrent.
(iii) Above the threshold frequency ν0, the maximum kinetic energy of the emitted photoelectrons increases linearly with the frequency of the incident radiation, but is independent of intensity of incident radiation.
(iv) The emission of photoelectron is an instantaneous process. There is no time lag between the irradiation of the metal surface and emission of photoelectron.
Click Here to Go To Top of The Page