Physics - Grade XII or Standard XII
Chapter 18: Atoms, Molecules, and Nuclei
Thomson’s model of atom:
Sir J. J. Thomson built his atom model - known as plum pudding model - using the following facts: (a) atom consists of electrons which are negatively charged tiny particles, (b) atom as a whole is electrically neutral, i.e., total electric charge in an atom is zero. According to Thomson, atom is spherical in shape, it is filled with positively charged matter, and negatively charged electrons are embedded in this matter. Total charge in atom is zero because negative charge of electrons nullifies positive charge of matter.
Geiger and Marsden Experiment:
Geiger and Marsden performed this experiment at the suggestion of Rutherford. They bomboarded very thin gold foil with stream of α-particles and observed the scattered α-particles. Notice that α-particle means nothing but a nucleus of helium atom. They found that (a) most of the α-particles passed undeviated, (b) about 0.14% scattered by more than 1o angle, (c) about 1 in 8000 deflected by more than 90o angle, (d) some α-particles were even bounced back with 180o angle. These results were in total disagreement with Thomson’s atom model. As positive charge in atom - according to Thomson’s atom model - is not concentrated at a single point but diffused throughout the atom, deviations of α-particles through large angles were surprising.
Click Here to Go To Top of The Page
Rutherford’s atom model:
Rutherford’s atom model has following properties: (a) atom is spherical in shape and at the center of atom there is a tiny, positively charged nucleus, (b) the total positive charge and entire mass (99.9%) of atom is confined in nucleus, (c) the nucleus is surrounded by negatively charged electrons, orbiting round the nucleus like planets round the Sun, thus centrifugal force acting on electrons due to orbiting balances the Coulombian force of attraction, (d) an atom is electrically neutral, the positive charge on nucleus is equal to the total negative charge of all the orbiting electrons, (e) nucleus is 100,000 times smaller than atom, it means atom is mostly empty.
Merits of Rutherford’s atom model:
It successfully explained the results of Geiger and Marsden experiment.
Shortcomings in Rutherford’s atom model:
(a) a law in electrodynamics tells us that accelerated electric charge must emit electromagnetic radiation. It means, the revolving electrons (which experience centripetal acceleration) must emit electromagnetic radiation, consequently must lose energy, and finally fall into nucleus. Thus Rutherford’s atom model cannot give us a stable atom. (b) it cannot explain origin of spectral lines in hydrogen spectrum.
Bohr’s atom model:
Niels Bohr proposed a new atom model for hydrogen based on classical mechanics, electrodynamics, and Planck’s quantum theory. He made three postulates (or assumptions) and on the basis of these postulates he built his atom model.
Click Here to Go To Top of The Page
Postulate 1 of Bohr atom model:
Electron in a hydrogen atom revolves in circular orbit around the nucleus with nucleus at the center of orbit. The Coulombian force of attraction between positive nucleus and negative electron plays the role of centripetal force of attraction. Thus, we have:
m v2/r = k e2/r2
where, m = mass of revolving electron, v = linear speed of electron, r = radius of circular orbit of electron, k = Coulomb's constant = 8.99 x 109 N.m2/C2, e = magnitude of charge on electron and nucleus, LHS of above equation represents centrifugal force, whereas RHS of above equation represents Coulombian force of attraction (i.e., centripetal force).
Postulate 2 of Bohr atom model:
The electron revolves around the nucleus only in those orbits for which the angular momentum of electron is integral multiple of h/(2π). Electron while moving in this orbit does not emit any electromagnetic radiation. These orbits are called quantized orbits or Bohr orbits. Thus, we have:
m v r = n h / (2 π)
LHS of above equation represents angular momentum of electron, whereas RHS of above equation represents integral multiple of h/(2π), n = positive integer = 1, 2, 3, This n is called principal quantum number, it denotes the serial number of orbit.
Click Here to Go To Top of The Page
Postulate 3 of Bohr atom model:
When electron jumps from orbit of higher energy to orbit of lower energy, it radiates energy in the form of quanta or photon. The energy of emitted photon is equal to the difference between energies of two orbits in which transition takes place. Thus, we have:
hν = En – Ep
where hν = energy of emitted photon, h = Planck’s constant, ν = frequency of photon, En = energy of electron in the nth orbit, Ep = energy of electron in the pth orbit, also n > p and En > Ep.
Radius of Bohr orbit:
Bohr orbit means an orbit in hydrogen atom. Radius of Bohr orbit is given as follows:
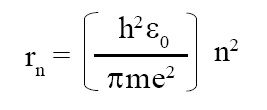
where r = radius of nth Bohr orbit.
Click Here to Go To Top of The Page
Energy of electron in Bohr orbit:
The energy of electron in Bohr orbit is given by the following expression:
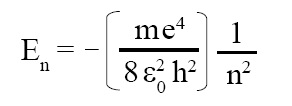
where symbols have usual meaning.
Characteristic spectrum of gas:
When a gas is heated or subjected to strong electric field or suitable radiation, it emits electromagnetic radiations of specific wavelengths. The spectrum of these radiations is called characteristic spectrum of that gas.
Hydrogen spectrum:
Hydrogen spectrum means characteristic spectrum of hydrogen gas. It was first observed by Balmer in 1885. He found four lines in this spectrum in the visible region. These lines are called Hα, Hβ, Hγ, and Hδ lines. However, there are more lines in hydrogen spectrum.
Click Here to Go To Top of The Page
Bohr’s formula for hydrogen spectrum:
Bohr provided following formula for hydrogen spec- trum. When electron from orbit m jumps to orbit p then photon of wavelength λ is emitted. This photon gives rise to spectral line:
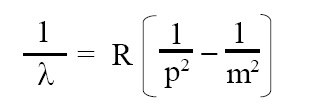
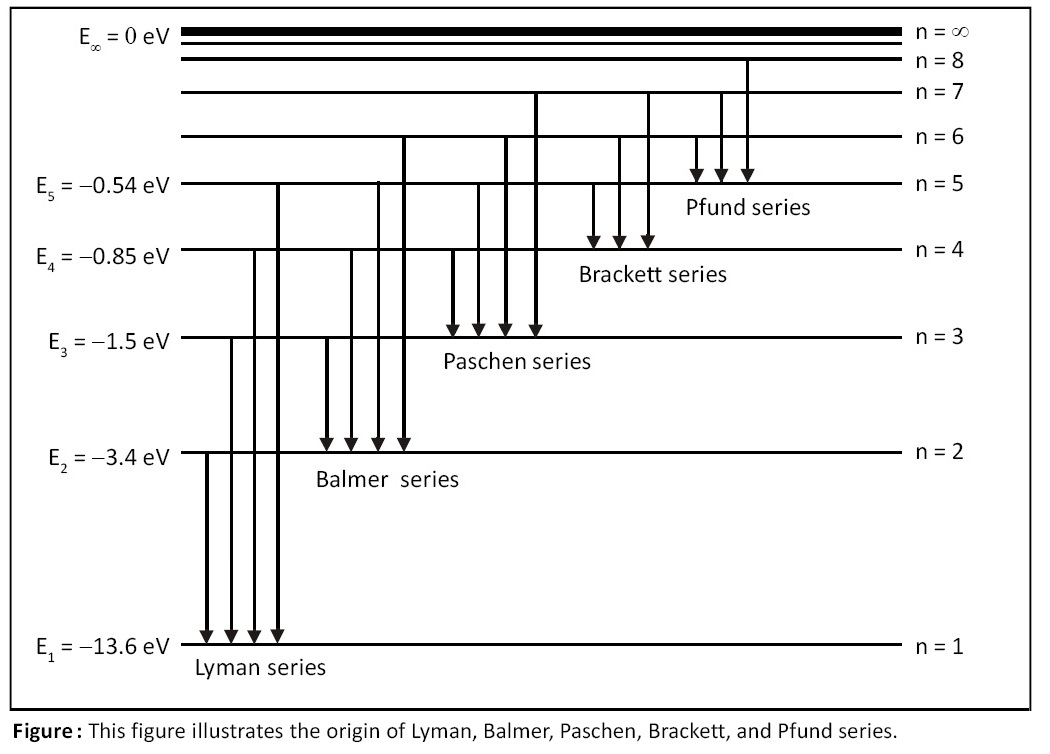
where λ = wavelength of spectral line, R = Rydberg’s constant = 1.093 × 107 m-1, n = principal quantum no. of outer orbit, p = principal quantum number of inner orbit. LHS of above equation (i.e., 1/λ) is called “wave number” and is denoted by "ν with overhead bar on it". This formula offers explanation for Lyman, Balmer, Paschen, Brackett, and Pfund series as follows:
Put p = 1 in this formula and you get Lyman series.
Put p = 2 in this formula and you get Balmer series.
Put p = 3 in this formula and you get Paschen series.
Put p = 4 in this formula and you get Brackett series.
Put p = 5 in this formula and you get Pfund series.
Click Here to Go To Top of The Page
Click Here to Go To Top of The Page
Nucleus:
Nucleus lies at the center of atom. It consists of protons and neutrons. About 99.9% mass of atom is actually mass of nucleus; rest of the mass (0.1%) is the mass of orbitting electrons. Mass of proton is almost the same as mass of neutron. Proton has 1 electronic positive charge on it. Neutron has zero charge.
Necleon:
Nucleon is a generic term that denotes proton as well as neutron (like the generic term parent that denotes father as well as mother). Generally letter “p” is used to denote proton and letter “n” is used to denote neutron.
Atomic number (Z):
The number of protons present in nucleus of an atom is called atomic number of that atom and is denoted by letter “Z.”
Mass number (A):
Total number of protons and neutrons present in the nucleus of an atom is called mass number of that atom and is denoted by letter “A.”
Click Here to Go To Top of The Page
Symbol of nucleus:
Nucleus is symbolically expressed as follows:
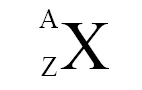
where A = mass number, Z = atomic number, X = chemical symbol of that element. For example, nuclei of gold (Au) and uranium (U) are denoted as follows:

Isotopes:
The nuclei having same number of protons (Z) but different number of neutrons (A – Z) are called isotopes.
Isotones:
The nuclei having same number of neutrons (A – Z) but different atomic numbers (Z) are called isotones.
Isobars:
The nuclei having same mass number (A) are called isobars.
Mass-Energy relation:
Einstein derived following mass energy relation:
E = mc2
where E = energy, m = mass, c = speed of light.
Click Here to Go To Top of The Page
Mass defect:
The mass of a nucleus is smaller than the sum of the masses of contituent nucleons in the free state. The difference between the actual mass of the nucleus and the sum of masses of constituents nucleons is called mass defect. Mass defect is given by the following formula:
Δm = [Zmp + (A – Z) mn] – M
where Δm = mass defect, Z = atomic number, mp = mass of proton, A = mass number, mn = mass of neutron, M = mass of nucleus.
Nuclear binding energy:
The amount of energy required to separate all the nucleons from the nucleus is called binding energy of the nucleus.
Click Here to Go To Top of The Page
Radioactivity:
The phenomenon of spontaneous emission of radiations from radioactive substance is known as radioactivity.
Radioactive decay:
Radioactivity is a nuclear phenomenon in which an unstable nucleus undergoes a decay which is known as radioactive decay.
Three types of radioactive decay : There are three types of radioactive decay as follows:
a)
α-decay (alpha-decay):
In alpha decay a helium nucleus 2He4 is emitted.
b)
β-decay (beta-decay):
In beta decay an electron (e–) is emitted.
c)
γ-decay (gamma-decay):
In gamma decay high energy photons are emitted.
Click Here to Go To Top of The Page
Properties of α-particles:
Properties of α-particles are as follows:
(a) It is nucleus of helium atom. It consists of 2 protons and 2 neutrons.
(b) As it is charged particle, it is deflected by electric and magnetic fields.
(c) The speed of emission of α-particles depend upon the nature of radioactive element. It varies from (1/10)th to (1/100)th of the speed of light.
(d) They affect photographic plate. They produce fluorescence.
(e) They ionize gas when passed through gas.
(f) The range of α-particles through air varies from 2.7 cm to 8.62 cm for thorium.
(g) They are scattered when incident on mica, aluminium, and gold foil.
(h) When an α-particle is emitted by an atom, its atomic number decreases by 2 and mass number decreases by 4. In the following example, uranium nucleus is conveted into thorium nucleus due to α-decay.
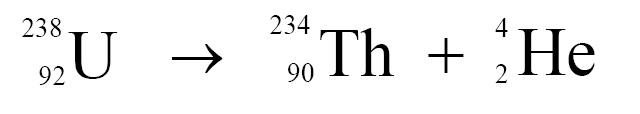
Click Here to Go To Top of The Page
Properties of β-particles:
Properties of β-particles are as follows:
(a) β-particle is a fast moving electron emitted by a nuclues.
(b) Its speed ranges from 1% to 99% of the speed of light.
(c) As it is a charged particle, it is deflected by electric and magnetic fields.
(d) They can ionize gas but its ionisation power is (1/100)th of that of α-particle.
(e) It is more penetrating than α-particle.
(f) Its range in air depends on its speed. A β-particle of 0.5 MeV has a range of 1m in air.
(g) When β-particle is radiated, the atomic number increases by 1 and mass number does not change, In following example, phosphorus nucleus is converted into sulfer nucleus due to β-decay.
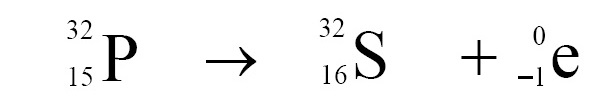
Click Here to Go To Top of The Page
Properties of γ-rays:
Properties of γ-rays are as follows:
(a) γ-rays are nothing but energetic photons. Photons originating from nucleus are called γ-rays.
(b) As γ-rays are photons, like other photons γ-rays are electrically neutral and are not affected by electric and magnetic fields.
(c) They affect photographic plate and produce fluorescence.
(d) They have very low ionization power, about (1/1000)th of that of α-particles.
(e) Thy have high penetration power and can pass through 25 cm thick iron plates.
(f) They are diffracted by crystals.
Radioactive disintegration:
The spontaneous breaking of a nucleus is known as radioactive disintegration.
Decay law:
The number of nuclei undergoing the decay per unit time is proportional to the number of unchanged nuclei present at that instant.
Click Here to Go To Top of The Page
Decay constant (λ):
Definition 1: Decay constant is defined as ratio of the amount of substance disintegrated per unit time to amount of substance present at that time.
Definition 2: Decay constant can be defined as the reciprocal of time duration t in which the 37% of the substance disintegrates.
Half life period (T):
Half life period (T) of radioactive substance is defined as the time in which the half of the substance disintegrates.
Nuclear fission:
When a heavy and unstable nucleus is bombarded with neutrons, it splits up into two separate nuclei. This process is called nuclear fission. For example, when uranium nucleus is bombarded with neutrons, it splits into barium and krypton nuclei, as follows:
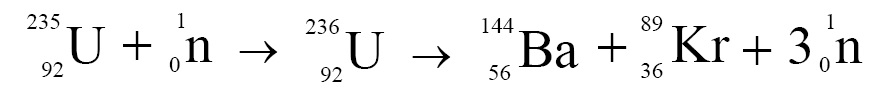
Notice that in this fission reaction, a neutron is bombarded on U235 nucleus, latter absorbs this neutron and is converted into U236 isotope of uranium. As U236 isotope of uranium is highly unstable it breaks into a barium nucleus, a krypton nucleus and the three neutrons. These neutrons, being energetic, can induce more fission reactions, and thus a self sustained chain reaction can be achieved that is used in nuclear bombs.
Click Here to Go To Top of The Page